
Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially Your Infringement Notice may be forwarded to the party that made the content available or to third parties such Means of the most recent email address, if any, provided by such party to Varsity Tutors. Infringement Notice, it will make a good faith attempt to contact the party that made such content available by

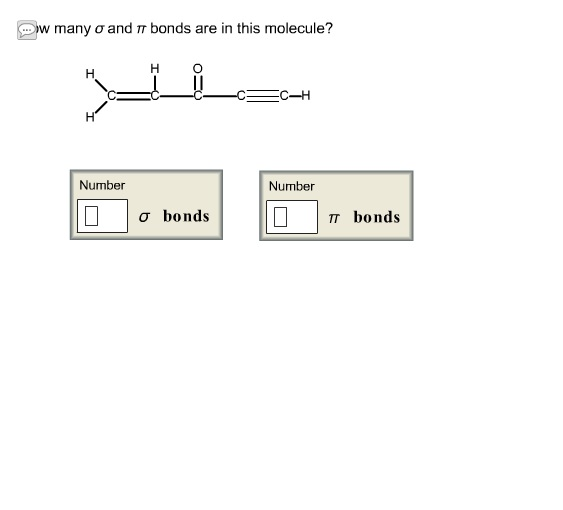
If Varsity Tutors takes action in response to Information described below to the designated agent listed below. Or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one Each of these orbitals will take place in the three sigma bonds that the carbon atom has. These three orbitals can hybridize to become three orbitals. Consequently, we have one s orbital and two p orbitals left over. Thus, one out of the three p orbitals from the carbon will be used towards contributing to the pi bond with the oxygen. In order to have a pi bond, there must be p orbital overlap. Thus, in total, this carbon atom will have three sigma bonds and one pi bond. Each of the two single bonds that carbon has to the other atoms will also be sigma bonds, because these are both single bonds.

Let's also recall that a double bond consists of one sigma bond and one pi bond. In addition, the carbon also has a single bond to each of two other atoms, neither of which are hydrogen. For this question, we're asked to identify which kind of hydridization, if any, a carbonyl carbon of a ketone group will have.įirst, let's recall that a ketone group is a functional group in which a carbon atom contains a double bond to an oxygen atom.


 0 kommentar(er)
0 kommentar(er)
